Peak Picking in 4D Spectrum with POKY
Overview
The general workflow of this tutorial starts with referencing and converting the spectra to POKY/Sparky format. Then, we will create 2D projections from the 4D spectrum, which will help us reference it properly.
For peak picking, we will follow a systematic strategy that, although more involved, will allow for higher precision in identifying peaks in the 4D spectrum while minimizing noise. Since the 2D projections are derived directly from the 4D spectrum, they provide a more accurate reference than the HSQC spectra, whose peak centers may deviate slightly from those in the projections.
To ensure accurate peak selection, we will use the 2D projections as intermediate reference points for restricted peak picking in the 4D spectrum. The workflow is as follows:
- Overlay the projections onto the corresponding HSQC spectra
- Identify peak centers by using the HSQC spectra as references
- Use these peak centers to perform restricted peak picking in the 4D spectrum
- Unfold or unalias peaks as necessary
- Remove noise peaks from the 4D spectrum
By following this approach, we ensure that the final set of picked peaks in the 4D spectrum is as accurate and noise-free as possible.
Prerequisites
- Installation of POKY or NMRFAM-Sparky; license for POKY.
- Copy the enhanced Restricted Peak Pick POKY plugin that reads the tolerance values from the peak’s “Note” field from
nmr_tutorials/SPARKY_and_POKY/POKY/scripts/restrictedpick.pyto yourPOKY/poky_linux/modules/poky/folder. - Access to the specified CA2 protein 4D HCNH NOESY, 15N HSQC and 13C HSQC spectrum files.
Steps
Step 1. Reference the HSQC Spectra in Topspin
Follow the instructions to reference the 1H-15N and 1H-13C HSQC in Topspin with BioTop.
2.2 Convert Spectra to UCSF Format
Enter the directory where each spectrum is saved in Bruker format and run bruk2ucsf from there—running it from another directory will fail.
For example, to convert the 1H-15N, 1H-13C HSQC spectra, and the 4D HCNH NOESY:
bruk2ucsf_run 6/pdata/1/2rr /srv/NMR/Peak_Picking/Nanoluc/15N_HSQC.ucsf
bruk2ucsf_run 7/pdata/1/2rr /srv/NMR/Peak_Picking/Nanoluc/13C_HSQC.ucsf
bruk2ucsf_run 5/pdata/1/4rrr /srv/NMR/Peak_Picking/Nanoluc/4D_HCNH_NOESY.ucsf
Note: You can also convert the spectra from Bruker to UCSF format in POKY/Sparky, but you cannot rename the axes in that process.
2.3 Rename Axes
Rename the axes in the 1H-15N and 1H-13C HSQC spectra:
ucsfdata -a1 N -a2 HN 15N_HSQC.ucsf
ucsfdata -a1 C -a2 HC 13C_HSQC.ucsf
Print the axis values of the 4D HCNH NOESY:
ucsfdata 4D_HCNH_NOESY.ucsf
Example output:
axis w1 w2 w3 w4
nucleus 1H 13C 15N 1H
matrix size 256 256 256 416
block size 8 8 8 13
upfield ppm 1.194 6.301 101.402 5.279
downfield ppm 8.208 73.001 133.002 10.622
spectrum width Hz 6666.667 15939.978 3043.445 5078.125
transmitter MHz 950.374 238.980 96.311 950.374
From the upfield and downfield rows, you can guess which axis is HC and which is HN. In this example, the following
command renames them properly—amidic protons have higher shift values than the aliphatic protons:
ucsfdata -a1 HC -a2 C -a3 N -a4 HN 4D_HCNH_NOESY.ucsf
IMPORTANT: Make sure that axes are named consistently in all spectra; otherwise, you will encounter problems during peak picking.
2.4 Create C-HC and N-HN Projections
For a detailed tutorial, see Create_2D_projections_from_4D_spectrum.
Briefly, extract the N-HN projection from the 4D HCNH NOESY. You may need to adjust the -p[1-4] values according
to your 4D spectrum dimension order:
ucsfdata -p1 -r -o C-N-HN.ucsf 4D_HCNH_NOESY.ucsf
ucsfdata -p1 -r -o 2D_N-HN_proj.ucsf C-N-HN.ucsf
Similarly, for the C-HC projection:
ucsfdata -p4 -r -o HC-C-N.ucsf 4D_HCNH_NOESY.ucsf
ucsfdata -p3 -r -o 2D_HC-C_proj.ucsf HC-C-N.ucsf
Step 3. Loading the Spectra
Load the spectra
- Open the UCSF files with the
focommand (make sure to display Poky Spectrum type of files in the pop-up browser), navigate to the folder, and select your spectra. Alternatively, you can copy the full path to each spectrum (for example,realpath 4D_HCNH_NOESY.ucsfin the Shell) and paste it into the pop-up browser. - Do the same with the 2D projections and the
HSQCspectra. - Use
xato show the nucleus types on the axes.,xrto roll the axes andxxto transpose them. - Fix the aspect ratio by hitting
vtand increasing the Aspect (ppm) for example to12, and then Apply.
Step 4. Adjusting the Spectra
Synchronize Spectra
- Click
ytto synchronize theNaxes of the1H-15N HSQCand4D_HCNH_NOESYfirst, and then synchronize theHNaxes of those same spectra. Remember to synchronize one axis at a time! - Do the same for the
1H-13C HSQCand4D_HCNH_NOESY, and then for bothHSQCspectra and the respective 2D projections.
Correct the contour levels and colors
- Type
vCto bring up the contour level control scrollbars and adjust the contour levels. - Type
ecto bring up the easy contour dialog allowing you to adjust all loaded spectra, including their colors. - If you double-click on a spectrum in the dialog it will open up the contour level dialog (equivalent to the
ctcommand).
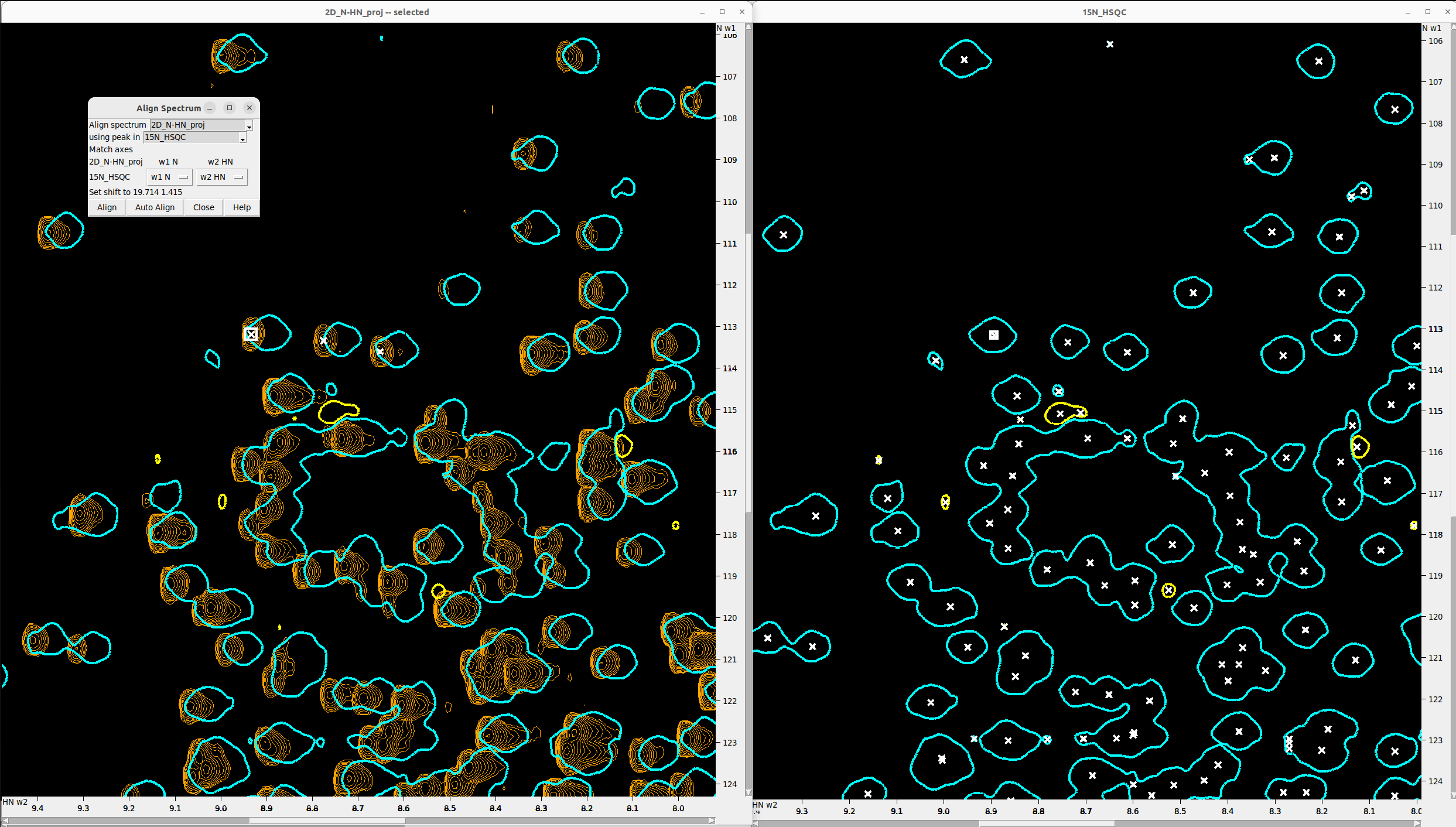
Align the 2D_N-HN_proj to the 15N_HSQC
- The 4D axes are usually completely off and must be aligned to the reference HSQC spectra. To achieve this, use the 2D projections.
- Hit
olto overlay2D_N-HN_proj.ucsfonto15N_HSQC. - You may reduce the contour number to 1 for one of the two spectra for better visibility. Also hit
ozto increse the contour thickness and the peak marker thickness. - Manually pick the most “trustworthy” peak in the
15N_HSQC(F8to enter peak picking mode,F1to exit it) and find the same peak in the 4D. - Type
al, and in the pop-up:- Set Align spectrum to
2D_N-HN_proj - Set using peak in to
15N_HSQC - The axes should match, thanks to the renaming we did earlier.
- Now hold the
Shiftkey and select one “trustworthy” peak in each spectrum for alignment. - Hit Align.
- Alternatively pick a set of matching peaks (button
F8) in both spectra and click the Auto align option (slower and doesn’t always work well).
- Set Align spectrum to
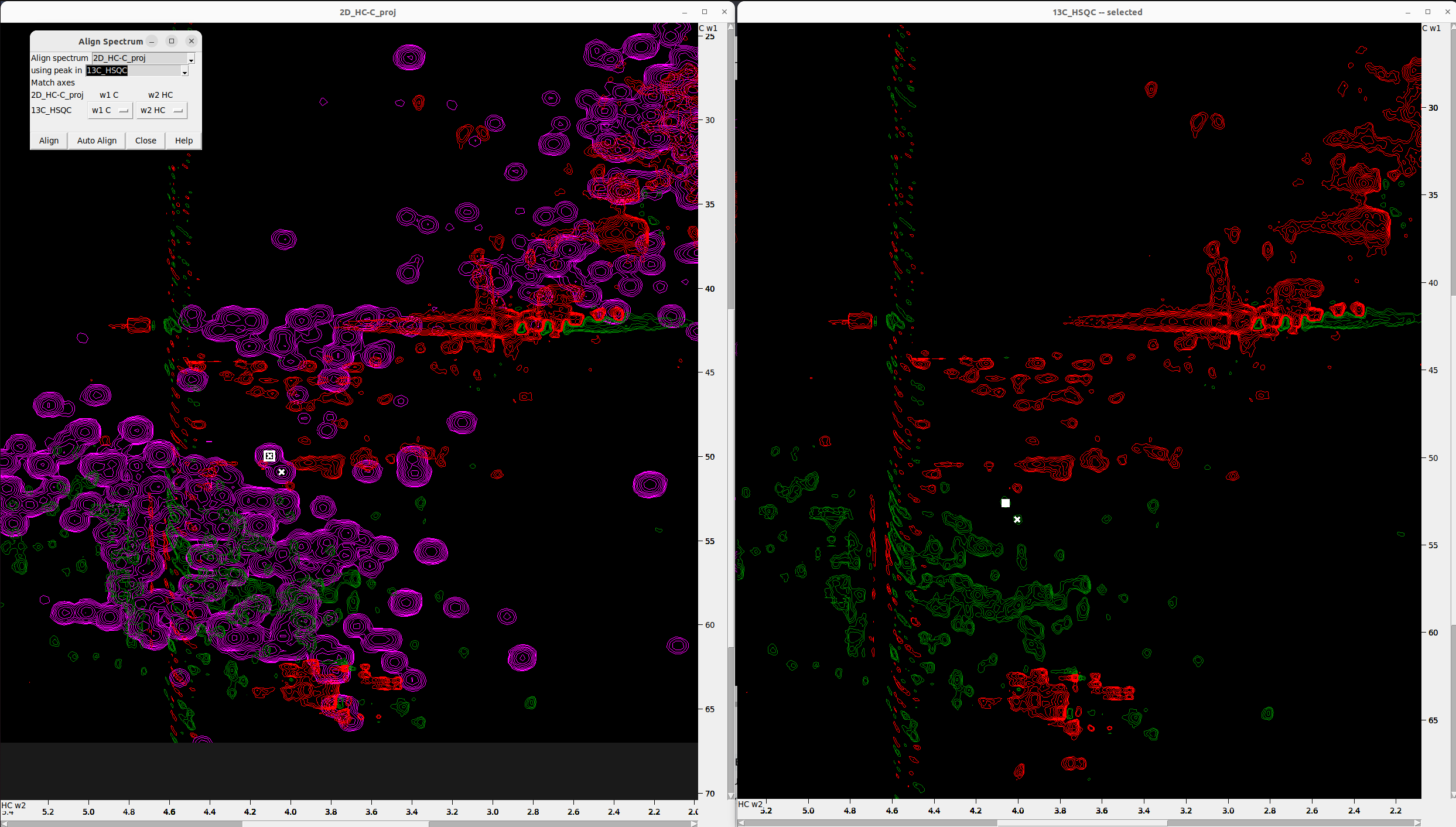
Align the 2D_HC-C_proj to the 13C_HSQC
Follow the same procedure described in the previous step.
Reference the 4D_HCNH_NOESY
- Hit
stand copy the shift values from the aligned2D_N-HN_projto4D_HCNH_NOESY. - Make sure that every time you copy a shift value from one spectrum to the other, you click Apply, or else the value is not saved.
- Similarly, copy the shift values from the aligned
2D_HC-C_projto4D_HCNH_NOESY. - When you finish, click OK, and the
4D_HCNH_NOESYwill be referenced!
Step 5. Peak Picking
Adjusting Contour Levels and Preparing Reference Peaks
- Adjust the contour levels (both positive and negative) in the
15N HSQCspectrum to optimize peak visibility. - Press
F8to enter peak picking mode and select all visible peaks. - Use the following Python function to estimate the expected number of N-H (in-phase) peaks from the amino acid sequence in the multiplicity edited 15N HSQC:
def estimate_15N_hsqc_peaks(sequence: str) -> int: # Count backbone amides = total residues minus any prolines and the N-terminus backbone_peaks = sum(1 for aa in sequence if aa != 'P') -1 # Count side-chain peaks from R (NE–HE), K (NZ–QZ), W (NE1–HE1), and H (ND1-HD1) sidechain_peaks = sequence.count('R') + sequence.count('K') + sequence.count('W') + sequence.count('H') return backbone_peaks + sidechain_peaks - Gradually increase or decrease the contour levels until the number of in-phase (positive intensity) picked peaks is approximately the expected number of N-H peaks (292 for CA2 protein).
I set the contour level to 1.4e+06, which captured 274 in-phase peaks, as below that a lot of noise peaks were emerging.
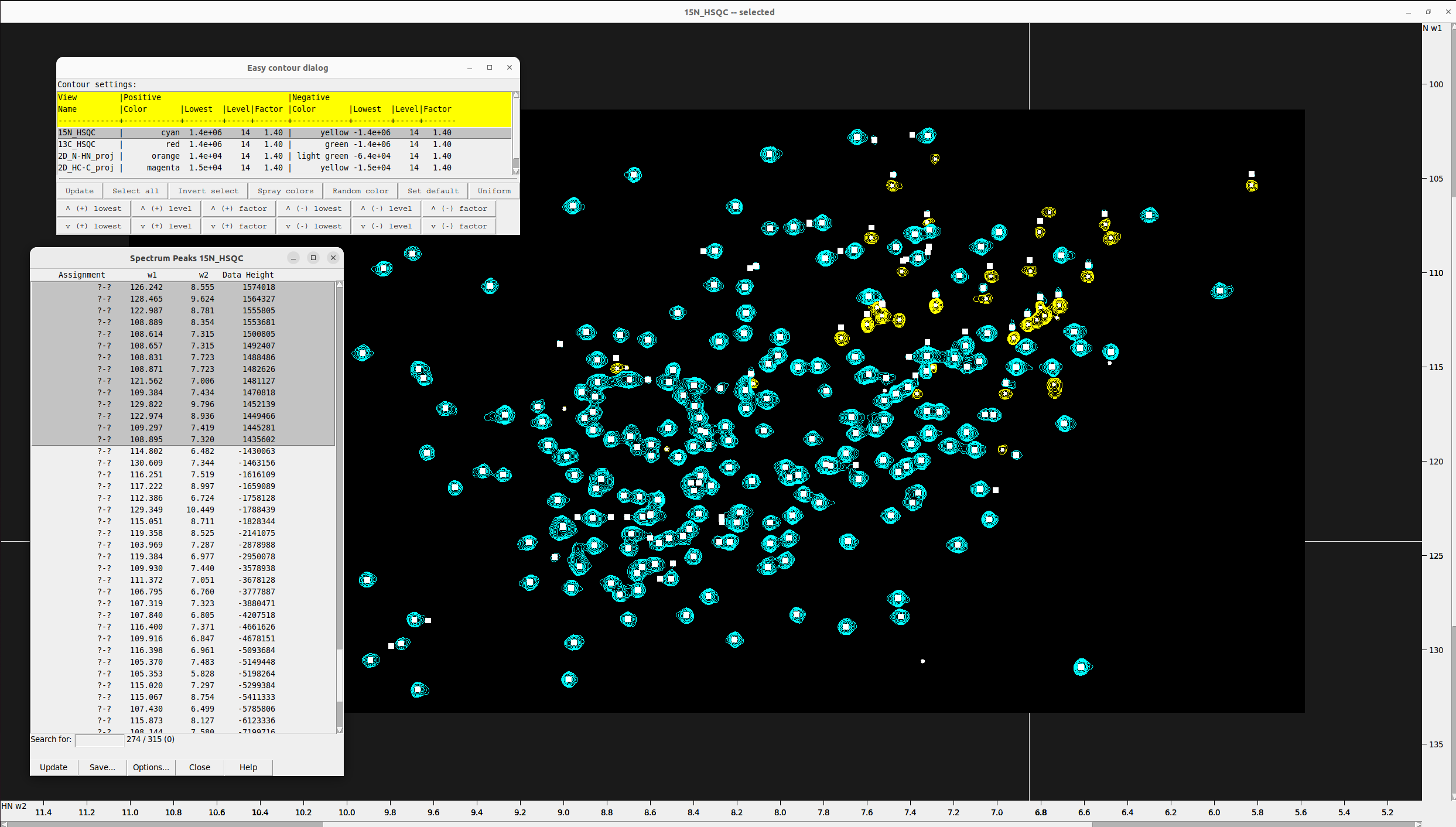
- Press
ltto open the peak list and export the peak list from the15N HSQCspectrum by saving it to a file. - Switch to the
2D N-HN projection, hitoland overlay15N_HSQCto2D_N-HN_proj. - Delete all current peaks from the
2D N-HN projection. - Press
rpto load the saved peak list from the15N HSQC. Alternatively, just copy (Ctrl+c)-paste (Ctrl+v) from one spectrum to the other. - Press
pato select all peaks. - Press
pcto adjust the peak positions to the contour centers of the2D projection. - Go through all the peak markers, visualize them and adjust their position manually to be at the center of the contours hills
in the
2D_N-HN_proj. To help you spot the overlapping peak markers, hitlt, sort by frequency and go through the list search for peaks with identical coordinates. Double-click on them and adjust their position based on the15N_HSQC.- At the end count the peak markers in both spectra usinglt. They must be the same. If not, check for peaks with the same coordinates - they are seen as one. - These peaks will be used as reference points for restricted peak picking in the
4D spectrumlater on.
Below you see the final peak selection on the N-HN_proj (orange) adjusted to the peak centers. The 15N_HSQC is
overlaid with cyan and yellow color. Notice that some peaks that were not present in the N-HN_proj (less sensitive experiment)
were added from the 15N_HSQC and the inverse (although much fewer peaks were added like this).
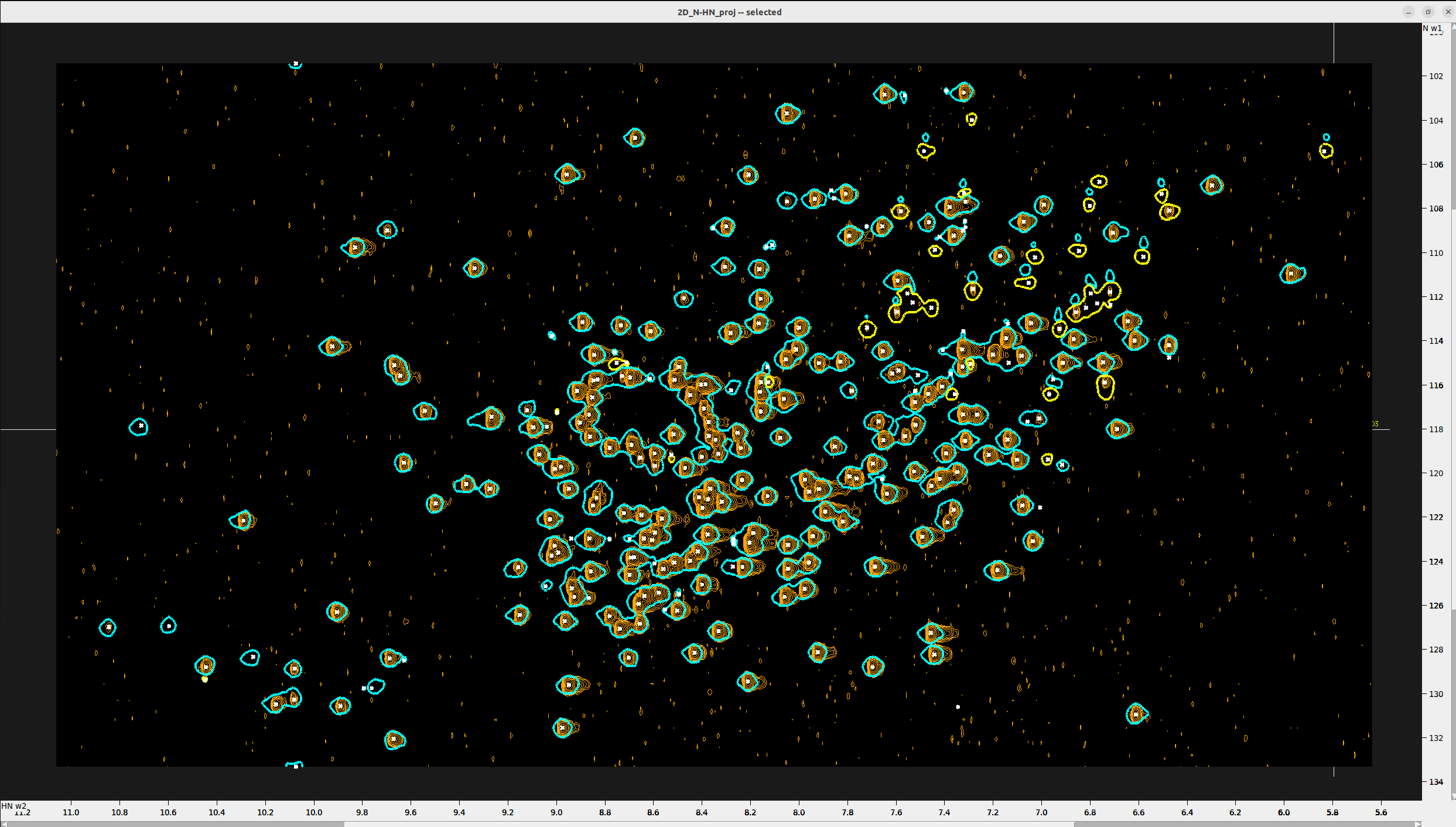
#——————————————
Pick all peaks in the HC-C projection that match with reference 1H-13C HSQC
Since 1H-13C HSQC is very crowded and is not ideal for setting landmarks for restricted peak selection, we will use it in
combinations ith the 2D_HC-C_proj. Overlay the two spectra and select with F8 all the peaks in 2D_HC-C_proj. The add manually
the peaks that you believe are within the general boundaries defined by the 1H-13C HSQC and are not noise - a bit of
intuition will be helpful here. Synchronizing the two spectra with yt and viewing them next to each other will help you.
In the following Figure I show how I add a new peak in the 2D_HC-C_proj based on the density of the 1H-13C HSQC.
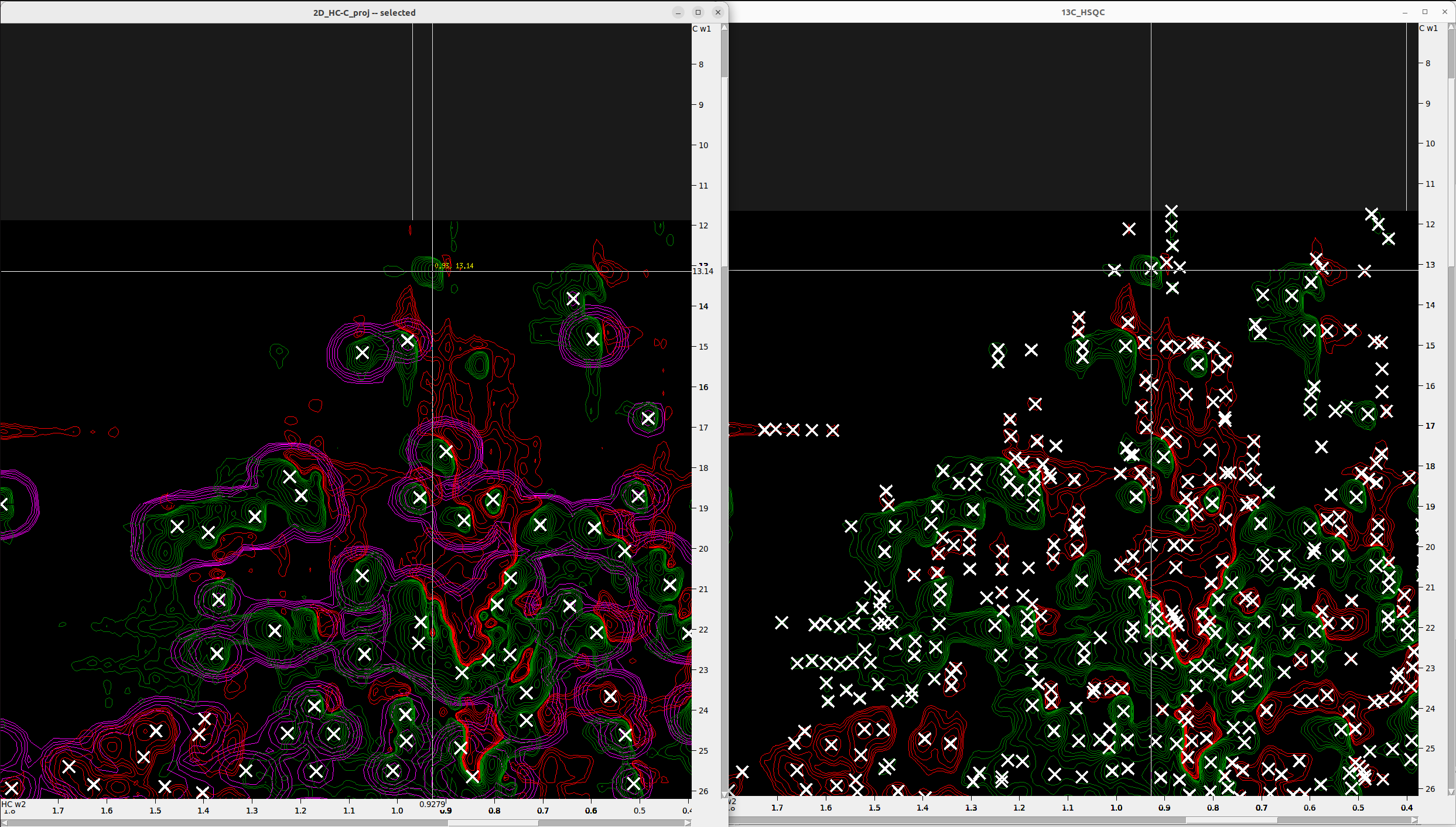
These peaks in 2D_HC-C_proj will be your landmarks for restrictive peak selection.
Optimizing Restricted Peak Picking for Higher Accuracy
- For more accurate restricted peak picking and to reduce noise, it is recommended to use different tolerances for peaks based on their radius.
- Open each projection spectrum, sort the peaks by data height, and start the lowest intensity peaks.
- Double-click on peaks in the
lttable to visualize them, hitmsto measure their radius in both axes and group them based on suitable tolerance values that will capture all corresponding peaks in the 4D spectrum within that region of the 2D plane. In general applying the same tolerance to all peaks with similar data height is a good strategy. - Once you decide about the tolerance values of a group of peaks, select them in the
ltwindow and hitntto save in a note the chosen tolerance values.
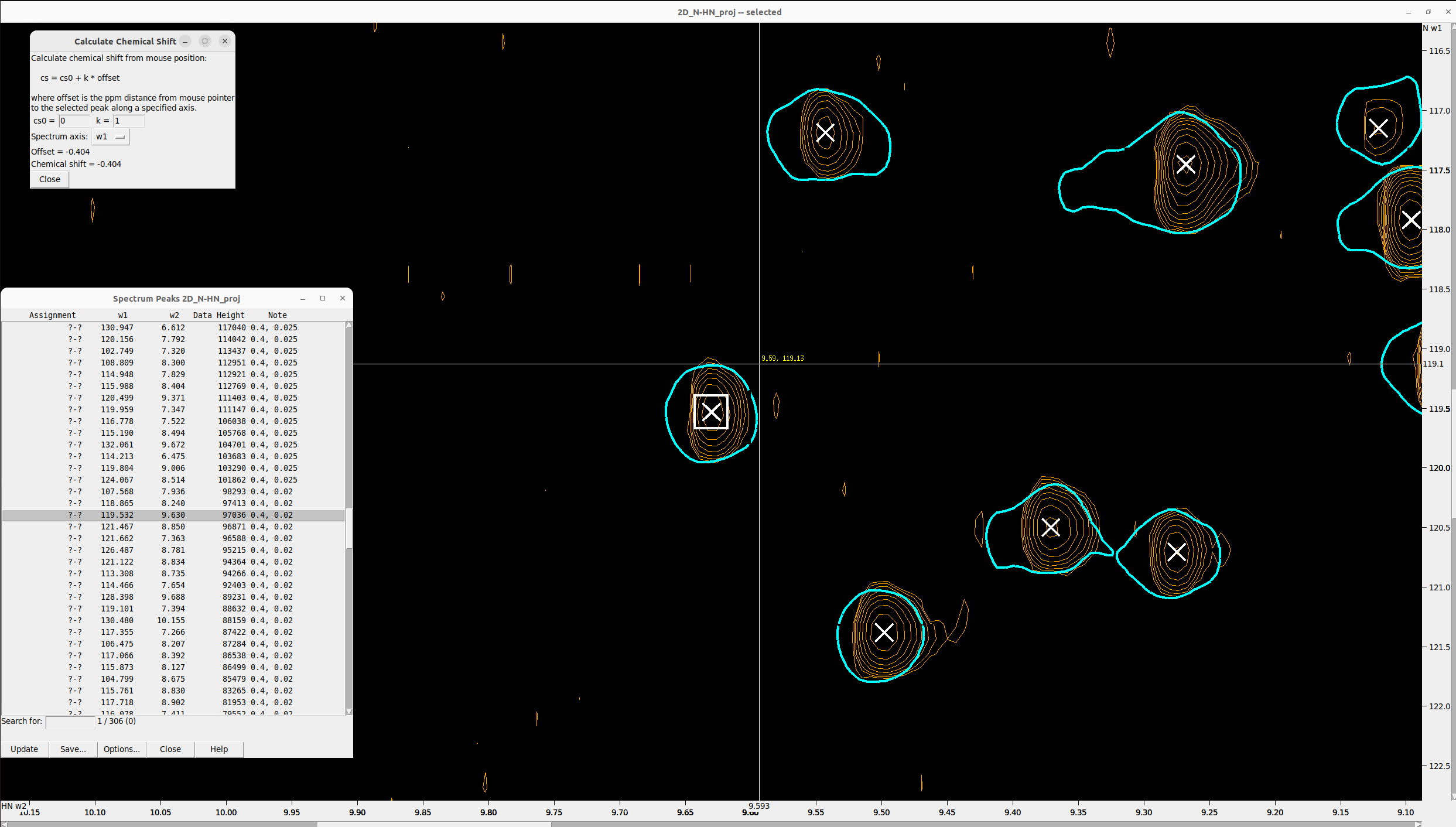
Since this protein is large, we will perform restricted peak picking in two rounds.
This is necessary because the screen updates every time a picking cycle completes, and for large proteins, this eventually becomes terribly slow.
- First, select approximately half of the peaks in the N-HN projection.
- Press
krto open the Restricted Peak Picking window. Make sure you copied our enhanced plugin to your POKY distribution (see Prerequisites)! - Activate the toggle options:
Use selected peaks onlyUse tolerance values from note
- Under Find peaks, select the 4D NOESY spectrum.
- Under Using peaks in, select the N-HN projection.
- Click the Pick Peaks button.
- Once it finishes, repeat restricted peak picking, this time:
- Set Using peaks in to the HC-C projection
- Deactivate the toggle
Use selected peaks only - Click the Select Peaks button.
- Then, switch to the 4D NOESY window, press
I(capital i), and then the Delete button to remove all irrelevant peaks. - The remaining peak list displayed on the HC-C plane of the 4D NOESY should now look clean.
- Press
pa(select all), thenNT, and write a word likematchedto mark these peaks. -
Click Apply.
- Now go back to the N-HN projection window.
- Select the remaining half of the peaks.
- Open the Restricted Peak Picking window again.
- Set Using peaks in to the N-HN projection.
- Activate both toggle options:
Use selected peaks onlyUse tolerance values from note
- Click the Pick Peaks button.
- Change Using peaks in to the HC-C projection.
- Deactivate
Use selected peaks only. - Click Select Peaks.
- Switch to the 4D NOESY window, press
NT, write the wordmatched, and click Apply.
⚠️ Important: Do not delete any peaks yet, as we did in the first round—otherwise, you will lose some of the peaks identified earlier.
- Press
ltto display the pick list in the 4D spectrum. - Click Options, sort the list by Note, activate the Note toggle, and click Apply.
- Our goal is to delete only those peaks that do not have a note. The good peaks are those with the word
matchedin the Note column. - Use the
Page UpandPage Downkeyboard buttons to scroll quickly and select large portions of peaks without notes. - Once a significant portion is selected, switch to the 4D NOESY window and press the Delete key to remove the selected irrelevant peaks.
💡 For large proteins with tens of thousands of peaks, it is recommended to delete them in two batches rather than all at once.
This is how the final peak selection on the HC-C plane of the 4D NOESY should look.
[FIGURE]
Step X. Unalias/Unfold 4D Peaks
Next, we will perform unaliasing/unfolding of peaks. For more details, please read the respective article.
Aliased Peaks usually occur in the ranges C < 25 ppm and HC > 3 ppm.
In this spectrum, we have some aliased peaks that appear on top.
- Press
F1to switch to selection mode, select the aliased peaks, and then pressa1to unalias them along the C axis (W1).
[FIGURE here]
Step X. Manual Refinement of 4D Peak List
Next, we will manually inspect all the peaks and remove those that are not located in density regions—neither of the HC-C projection nor of the N-HN projection.
- Type
foand load the 4D spectrum again, this time to a new window. - In the new window double-click
xrfollowed byxxto bring the N and HN axes into view. - Adjust the view by pressing
vtand increasing the aspect. - Press
vz, set the values to:- N and HN axes = 0
- C and HC axes = 9999
- Click Apply.
- You might need to adjust the peak sizes by typing
oz. - Hit
oland overlay:- First the N-HN projection onto the 4D
- Then the 15N HSQC onto the 4D
- Hit
stand rename this window to 4D_HCNH_NOESY - N-HN proj
You should now have two different views of the 4D spectrum:
- One showing the selected peaks on the HC-C plane
- The other showing the selected peaks on the N-HN plane
What you must do next is manually inspect the peaks and delete those not in density regions. This requires a
bit of intuition and a sharp eye. Unfortunately, it cannot be automated—it must be supervised manually by pressing st.
Discard the noise peaks using a S/N cutoff
- Hit
stand in the text box “noise as median of” write 10000 or another high number. - Click “Recompute” several times.
- If the “Estimated noise:” changes a lot, increase the “noise as median of” and repeat the process.
- Once you settle on an “Estimated noise:” value, open the peak list by hitting
lt, display the S/N and sort by Data Height. - Select all peaks with absolute S/N value less than the “Estimated noise:” and delete them. For stricter peak picking, you can set the cutoff to 3x or 5x the “Estimated noise:”.
** OPTIONAL: Discard the noise peaks using a weak peak as a reference**
- Overlay the
N-HN_projonto the15N_HSQCand increase the contour levels until noise peaks start to emerge. - Select a
15N_HSQCpeak with weak signal inN-HN_projand check its HC-C plane by switching to4D_HCNH_NOESYand hittingvc. - Review the picked peaks (previously found by
kr), adjusting the contour levels until all visible peaks are captured. - If you cannot achieve this, pick another weak peak in the
15N_HSQCand repeat the process. - Once you succeed, select the weakest picked peak and hit
ss. This command will select all 4D peaks that have lower intensity than the selected one. Typeltto check that. - Click Delete to remove them.
Note: This comparison is made using signed intensities: negative peaks with large absolute intensity will also be selected! Check them manually and deselect if needed: hold
Ctrland drag over the selected peaks with your mouse.
(^ There should be a GIF here showing large negative peaks.)
Step X. Exporting Peak Lists
Export Picked Peaks for 4D-GRAPHS
Go to the 4D peak list (type lt) and select the columns w1, w2, w3, w4, Data Height and Note. Click Apply, then Save….
Further editing of the projections and the HSQCs is needed.
Improve the Precision of the 13C HSQC Peak List using the 4D HCNH NOESY Peak List
- Once you have finalized the peak list from the HC-C projection, export it to a file.
- Press
foand reopen the 13C HSQC spectrum in a new window. - Press
stand rename that window to “13C HSQC - 4D Peak List”. - Hit
rp, toggle on Auto detect dimensions and load the 4D HCNH NOESY peak list. - Verify that the aliased/folded peaks are unaliased/unfolded, just as on the 4D spectrum (POKY does this automatically).
- Hit
ltand through the “Options” display the “Data Height”. - Export the updated peak list to a new file named
13C_HSQC_with_4D_HCNH_NOESY_peaks.list.
We follow this approach because the 13C HSQC spectrum is very noisy, with large dispersion effects, meaning
that the peak centers deviate from those identified in 4D HCNH NOESY spectrum.
Consequently, since the entire assignment relies on the 4D spectrum, it is more accurate to use the peak
markers from it, but enhanced with the intensity signs present in the 13C HSQC spectrum.
The 13C HSQC spectrum provides information on whether a peak corresponds to a methylene group, which improves
both accuracy and coverage for chemical shift assignment in 4D-GRAPHS.
Improve the Precision of the 15N HSQC Peak List using the 2D N-HN projection Peak List
The 15N HSQC peak list for 4D-GRAPHS must have only one peak for each spin system, therefore we will apply the
previous trick but using the `*N-HN projection peak list instead of the 4D HCNH NOESY peak list.
Notes for Special Cases
Unaliasing Peaks in POKY
When you do restricted peak picking (kr) using as a reference Peaks that have not been unaliased or unfolded, POKY
will automatically check for possible aliased peaks. If the spectrum width of the source 2D is larger than that of the
nD (n = [3,4]), POKY will find and mark the peaks in the 3D as aliased.
However, BEWARE that when your reference peaks are aliased or unfolded, POKY won’t match the correct peaks in the target spectrum unless they are also unalias/unafolded. It may catch some peaks but they will be irrelevant. Therefore, do not unalias/unfold the peaks in the 2D HC-C and N-HN projections! Do the unaliasing/unfolding directly on the 4D HCNH NOESY.
Below are examples of the 13C-HSQC spectra with aliased peaks (in yellow boxes):
| Protein 1 | Example 13C-HSQC - Protein 2 |
|---|---|
 |
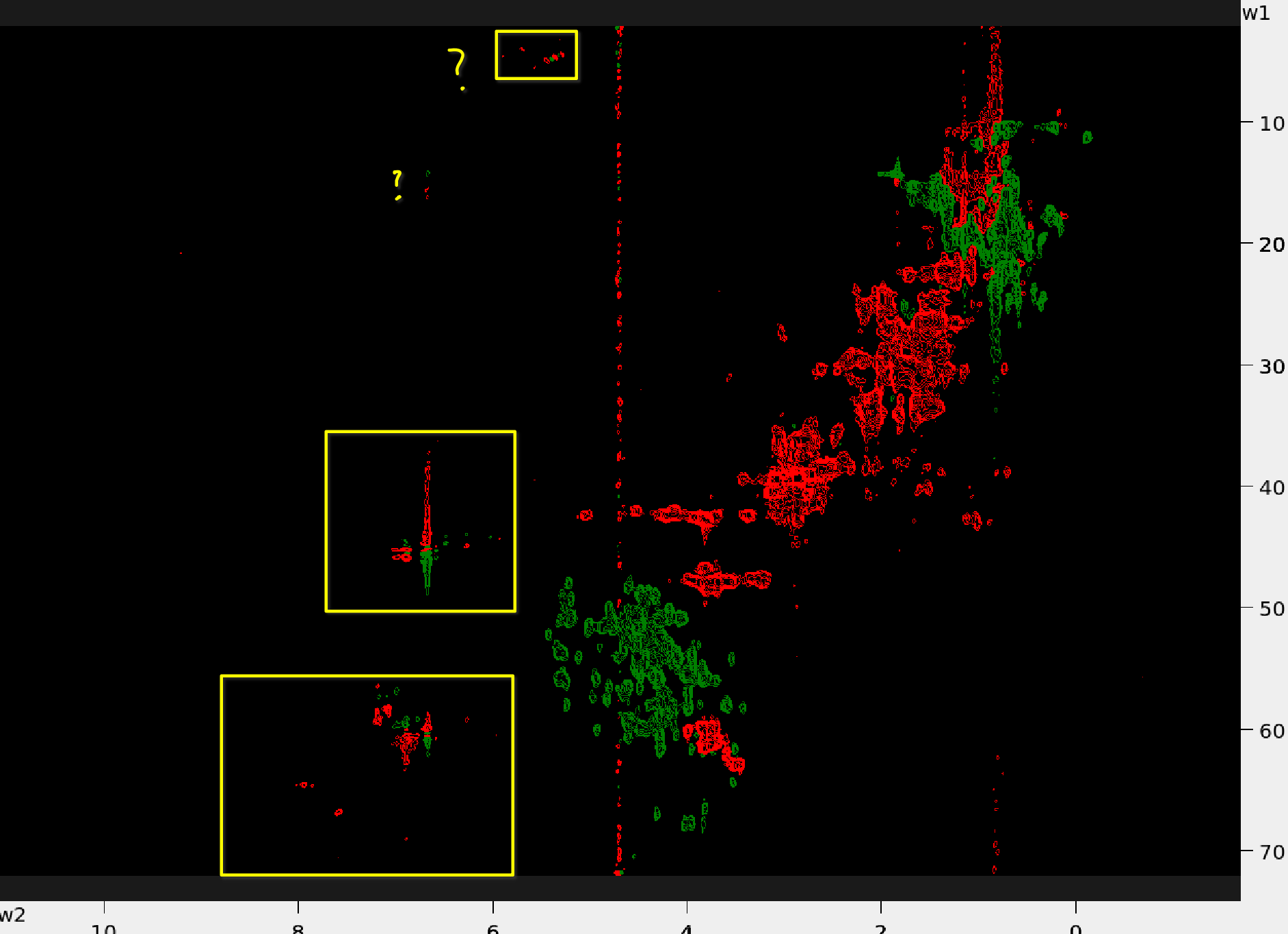 |
Authors
- Thomas Evangelidis
- Ekaterina Burakova